PALO ALTO, Calif., Sept. 22, 2022 /PR Newswire/ -- DyAnsys, Inc., has announced that Primary Relief, a percutaneous electrical neurostimulation device, has been approved to treat pain following a cardiac surgery.
The percutaneous electrical nerve stimulator (PENS) system can be used for up to three days for symptomatic relief of post-operative pain following cardiac surgery.
"This ground-breaking device allows for significant pain relief without the use of narcotics," said DyAnsys Chief Executive Srini Nageshwar. "By reducing or avoiding the use of opioids after surgery, the risk of addiction is reduced. We look forward to connecting with physicians and patients to make this option available after cardiac surgery among other applications."
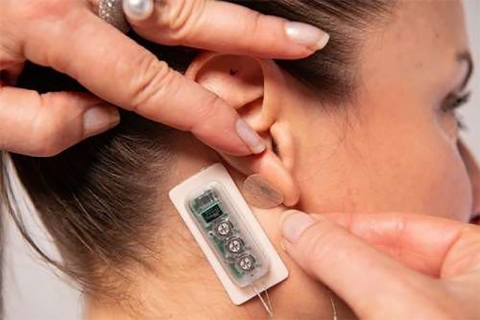
Primary Relief also has FDA clearance for use post Cesarean section (C-section) delivery. The wearable, battery-operated device is designed to administer periodic low level electrical pulses to the ear over 72 hours from the activation of the device. The electrical pulses are delivered to branches of the cranial nerves on the ear through a wire assembly and stimulation needles.
The effectiveness of the device for post-cardiac surgery was demonstrated in a single-centre, double arm, randomized, prospective study involving 60 patients. The analysis showed that minimally invasive nerve stimulation intervention using Primary Relief reduced the pain score compared to a placebo device. It also reduced the requirement for analgesics post surgery. The mean dosage of fentanyl required as a rescue analgesic in the postoperative period was only one-third of the control group. Only four patients treated with Primary Relief required diclofenac as a second rescue analgesic compared to 19 in the control group.
The nonclinical testing of Primary Relief device included biocompatibility testing, electrical safety (electromagnetic compatibility and safety), performance bench testing and software verification and validation.
DyAnsys also offers two companion PENS devices - First Relief, with FDA clearance for the treatment of diabetic neuropathic pain, and Drug Relief, with FDA clearance as an aid to drug withdrawal.
DyAnsys Inc. is a global company headquartered in California with subsidiaries in Switzerland and India . DyAnsys provides advanced medical diagnostic and monitoringsystems to clinicians in individual practices and hospitals. DyAnsys, ANSiscope. First Relief,Primary Relief and Drug Relief are registered trademarks, of DyAnsys, Inc.
Website: www.dyansys.com
For more information, contact Srini Nageshwar, (888) 950-4321
SOURCE DyAnsys Inc.

