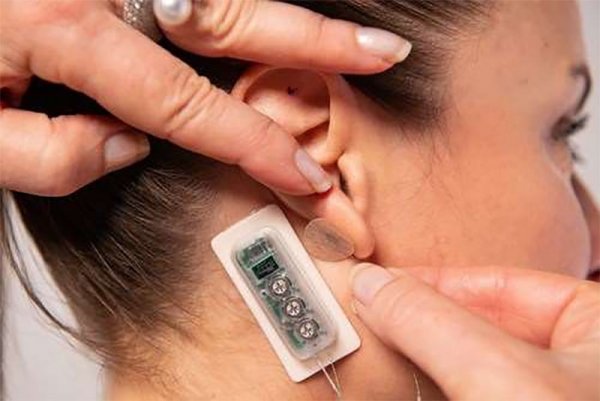
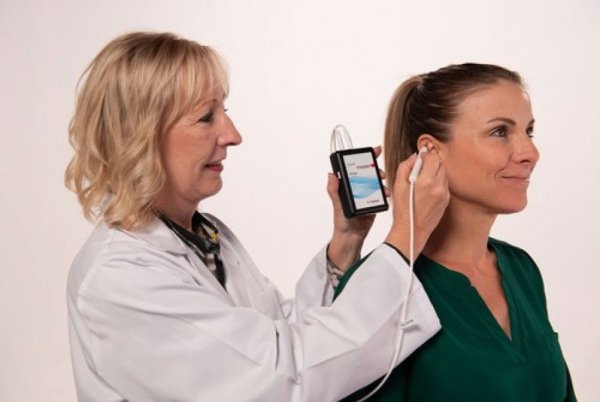
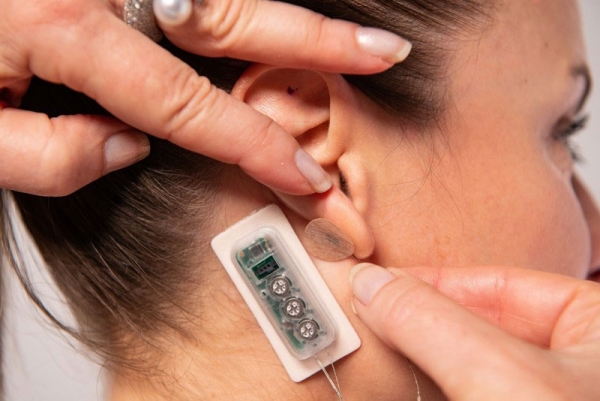
PALO ALTO, Calif., Sept. 22, 2022 /PR Newswire/ -- DyAnsys, Inc., has announced that Primary Relief, a percutaneous electrical neurostimulation device, has been approved to treat pain following a cardiac surgery.
The percutaneous electrical nerve stimulator (PENS) system can be used for...